Cohort 3 Research Projects
Amar Mohammed – Solar-driven Plasmonic Catalysis
Supervisor: Dr John Errington
This project addresses the major challenges associated with precise manipulation of interfaces for atom/charge transfer in catalysis and energy conversion, where high priority goals include visible-light plasmonic catalysis and high selectivity in e.g. CO2 conversion to MeOH. A range of new binary and ternary catalytic materials prepared from polyoxometalate-stabilised metal nanoparticles (POM@MNP) and polymeric carbon nitride (PCN) will build on previous work of the remarkable synergy in POM@Ru thermal, photo- and electro-catalysis.
Underpinning the project is the synthesis of heterometal-substituted polyoxometalates (POMs) as tunable, single-atom, molecular metal oxide catalysts. These will be used to prepare POM@AuNP and POM@CuNP binary systems in order to precisely locate reactive metal sites (e.g. Ti, Zr, V, Cr, Mn, Fe, Co, Ni, Zn) at the metal-oxide/metal interface. Ternary materials obtained by supporting these POM@MNP on PCN will provide additional functionality derived from the photo- and electro-active PCN. POM/MNP interfaces will be analysed by high resolution electron microscopy and the distribution of NPs on PCN will be determined using electron tomography. Thermal and photocatalytic experiments will be studied by time-resolved spectroscopy and supplemented by studies of ultrafast charge extraction dynamics. Charge transfer at POM/MNP, POM/PCN, MNP/PCN and POM@MNP/PCN interfaces will be key to exploiting the unique photo physics of the new binary and ternary materials for visible light plasmonic catalysis. Fine tuning the properties of these multicomponent catalysts will require a deeper understanding of these systems, and this will be aided by DFT computational modelling studies.
Precise manipulation of interfaces at the atomic level will improve activity and selectivity and enable catalyst design for new processes, providing better capabilities for CO2 utilisation and related energy conversion and storage.
Divyabhan Duggal – Low-Carbon Engineering Framework for a Resilient Water Network Using Renewable Energy and Storage Integration
Supervisor: Dr Haimeng Wu
This is an industry-based research project partnered with Northumbrian Water. A water utility company’s functions range from a variety of processes such as supplying water to their consumers, to carrying out wastewater treatment. Industrial processes such as these require high energy consumption. Although this energy can be supplied by the local utility grids however, the grid electricity is not completely from renewable sources. Northumbrian Water Group (NWG) is already fulfilling a major part of their energy needs using renewable energy. This has also allowed them to commit to become a carbon-neutral company by the year 2027. This project is also a part of NWG’s efforts towards becoming fully carbon neutral.
The aim of the project is to design an engineering framework for Northumbrian Water that integrates renewable energy technologies and energy storage systems for the carbon-neutral functioning of the company. The project targets at increasing the energy efficiency of the water network and in doing so, design a sustainable engineering framework which will help Northumbrian Water reduce its carbon emissions and provide economical and other benefits. The approach taken to tackle this problem, will be to identify and evaluate the opportunity(s) for incorporating renewable energy generation and storage systems in the current engineering framework. These could include a hybrid system consisting of solar photovoltaics, electric vehicles, and energy storage systems. These small-scale distributed technologies, when working in conjunction, can be optimized to form a smart water network. This smart water network will facilitate carbon savings and provide other valuable benefits such as improved power quality and efficient demand management for reduced interaction with the national electricity grid. Additionally, an improved operational efficiency might also be achieved via remote monitoring systems and the Internet of Things (IoT) platform.
A low-carbon engineering framework will be designed as a result of this project. Based on dynamic tracking in real-time, the designed framework may allow the users to be informed of operating decisions. Through the project, the efficiency of the overall system is aimed at for improvement, while also focusing at increasing the resilience of the water network for sustainable functioning of the company. The project falls mainly under the Energy theme of EPSRC but explores certain other EPSRC themes of research such as Artificial Intelligence and Engineering.
Andrew Thompson – Development of Graphene Silicon Lithium-ion battery Anodes
Supervisor: Prof Ahmed Elmarakbi
This project is in collaboration with Applied Graphene Materials UK Ltd (AGM). It focuses on the synthesis of a graphene-silica composite for anodes within lithium-ion batteries and the advancement of industrial-scale production.
With an ever-present pressure and awareness for sustainability and net-zero carbon emissions, our reliance on hydrocarbons must be replaced with that of renewable energy sources. However, the intermittency of renewables and inability to regulate energy production to meet peak demands force the requirement to improve energy-storage solutions. Away from the energy grid, the UK government aims to ban the production of internal combustion engine vehicles by 2030. This deadline causes the need to improve battery performance to meet consumer expectations for matters such as range, lifespan and charge rate.
In recent years, lithium-ion batteries have been demonstrated as the most promising energy-storage devices due to their high working voltage and energy densities (150Wh/kg), long life cycle and safe performance for consumer goods. Silicon-based anodes have recently become an attractive replacement for traditionally low performing graphite anodes, due to their extremely high theoretical capacity for lithium (4200 mAhg-1) and low working voltage (~0.2VvsLi/Li+). However, silicon suffers from poor electrical conductivity, excessive volume expansion (~300%) and structural deformation with the introduction of lithium. The latter two result in a considerable reduction in capacity over a multi-cycle period (upwards of 40% after the first cycle alone).
The introduction of graphene coatings and nanostructures around silica molecules is the most promising solution to overcome silicon’s limitations. The coatings/nanostructures aim to absorb the observed expansion and reduce the severity of fracturing (Figure 1), all whilst having the additional benefit of introducing graphene’s high electrical conductivity.
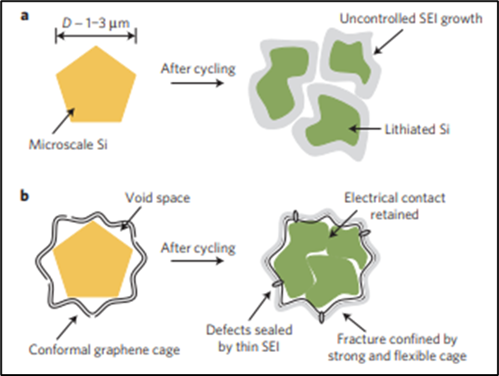
However, the synthesis of graphene-silica anodes, alone, is a very hot topic and widely covered by previous research. What this project aims to achieve is the novel enhancement of a scalable technique that can be replicated in industrial-scale production.
With this aim, multiple objectives must be achieved throughout the project:
- To synthesize graphene-silica composites and aerogels with AGM, through techniques such as chemical vapour deposition (CVD) and freeze-drying, to produce an effective battery anode material.
- To characterize the composites through multiple full-cycle (charge and discharge) periods and record the capacity retention and coulombic efficiency throughout.
- To characterize the composites by observing the extent of silica fracturing over the course of multi-cycle periods.
- To characterize the crystallinity of the graphene nanostructures/coatings to better understand how the variance of graphene formation affects the performance of the anode and formation of the solid electrolyte interface (SEI).
- To evaluate the potential of the given manufacturing technique(s) based on the characterisation/efficiency of the composite, and the time and cost of the production technique.
- To investigate how the variable conditions of the synthesis process, such as working temperature, vapour density and the distance from the vapour outlet to the base silica material, affects the morphology of the graphene layer.
Glen Hebberd – Developing New Oxysulfides for Photocatalytic and Photovoltaic Applications
Supervisor: Dr Emma McCabe & Prof. Stewart Clark
Photovoltaics are materials that can generate an electric current in response to light exposure. Silicon is currently the major component in many domestic and industrial photovoltaic solar cells. Photocatalysts are materials that can catalyse a chemical process in response to light exposure. CdS is a compound which is photocatalytically active under solar irradiation, utilising the solar energy to facilitate: (1) the hydrogen evolution reaction (allowing hydrogen extraction from water); (2) the carbon dioxide reduction reaction (allowing the breakdown of CO2 into usable fuels); (3) the degradation of environmental pollutants.1 Improving photovoltaic and photocatalytic materials therefore has significant environmental and financial advantages; giving rise to the quest to seek material families that can exploit as much of the solar radiation spectrum as possible.
In the field of energy materials, oxide materials are often unable to effectively exploit much of the visible region of solar irradiation. This is due to the bandgaps of these materials being too large, typically unable to match the wavelengths of light in the visible region (400 -700 nm). Oxysulfide materials contain both oxide and sulfide anions and the presence of the sulfide anion tends to increase the energy of the top of the valence band, decreasing the band gap. This means that oxysulfide materials can often be tuned to have bandgaps well-matched to the visible spectrum, optimising their performance under solar irradiation. Based on these observations, this project focuses on the structure prediction, synthesis, and characterisation of new oxysulfides for photocatalytic and photovoltaic applications.
The main challenge of this project is to explore the under-developed structural chemistry of oxysulfides, generating new functional ceramics and inorganics in the process. This will lead to the development of some chemical structure-property relationships so that new oxysulfide materials can be effectively designed and synthesised. Materials within this family will be targeted for their properties, so that they could be used for real-world industrial energy applications. This is relevant to the energy sector as promising photocatalytic properties are sought after for applications in the electrolysis of water (to enable more efficient production of green hydrogen) and promising photovoltaic properties are required in the search for cheaper, more efficient solar cell materials.
The project will involve experimental work to prepare materials (mostly by solid state reaction) and characterisation by X-ray single-crystal or powder diffraction. Promising samples will be characterised further by diffuse reflectance, and by catalysis measurements with collaborators. The project will also involve experiments (e.g. neutron diffraction) at central facilities on a select few materials. This practical approach will be combined with computational density functional theory (DFT) methods to calculate the electronic structure of materials so that promising oxysulfide candidates for the aforementioned applications can be identified.
Exploring the following EPSRC research areas (as given in bold in above text):
| – Functional ceramics and inorganics | – Materials for energy applications |
| – Condensed matter: electronic structure | – Chemical structure |
| – Computational and theoretical chemistry |
References
1. L. Cheng, Q. Xiang, Y. Liao and H. Zhang, Energy Environ. Sci., 2018, 11, 1362-1391.
Lawrence Bruce – Closing the carbon loop with biomass-waste derived carbon quantum dots
Supervisor: Dr Elizabeth Gibson and Dr Anh Phan
Abstract
This project will focus on the photocatalytic reduction of carbon dioxide (CO2) to produce sustainable fuels and feedstock, namely carbon monoxide (CO) and formic acid. To overcome the thermodynamic and kinetic challenges associated with CO2 reduction the enhancement of photon flux is essential. The optical properties of biomass-derived carbon quantum dots (CQDs) in a host matrix will be optimised to produce a printable luminescent solar concentrator (LSC). Waveguides will be produced by 3D printing with the architecture being designed to concentrate incident light towards a photocatalytic reactor. The overarching motivation of this project is to overcome the challenges associated with CO2 reduction to contribute to the circular carbon economy by the clean conversion of CO2 to fuels and feedstocks.
Project description
The current energetic landscape is changing in response to the threat that finite fossil fuels pose to the environmental and economic climate. In the face of the devastating consequences of global warming, innovative energy and fuel strategies are required. The reduction of CO2 is currently limited by the thermodynamic and kinetic challenges reflected by the high negative value of its free energy of formation (ΔGfo = -396 kJ mol-1). A large energy input is required to overcome the high overpotential associated with the formation of higher order products (CO2/HCOO– = -0.67 V (vs SHE) and CO2/CO = -0.52 V (vs SHE)). To yield sufficient current densities required for kinetically favourable reactions; the operating voltage of the electrode must account for the overpotential for both the reduction of CO2 (> 1 V) and water oxidation (~ 0.4 V). By the assembly of waveguides, concentrated light will be directed towards a photocatalytic reactor, the increased photon flux could be sufficient to efficiently reduce CO2.
A luminescent species will be suspended in the host matrix poly(methyl methacrylate), waveguides will be designed and produced by 3D printing or slot die coating. Waveguides trap a fraction of the emitted luminescence by internal reflection and the radiation energy is concentrated at the edge of the waveguide. The ideal luminescent solar concentrator should have:
- broad spectral absorption
- matched spectral response of the emitted photons and photocatalytic reactor
- a high photoluminescence quantum yield (PLQY)
- minimized re-adsorption of emitted photons – large Stokes shift
The photoluminescent (PL) properties of CQDs will be investigated. The effect of size, doping and surface functionalisation on PL will be screened against this criterion. Then the architectural design of waveguides on the concentration factor (C) of light achieved will be investigated.
Bottom-up synthesis methods provide greater flexibility to synthesise CQDs with varied size, morphology and surface functionality. Microwave pyrolysis and hydrothermal carbonisation (HTC) will be used to produce CQDs. By microwave pyrolysis, biomass is thermochemically decomposed to carbonaceous material which then can be activated by microwave heating. HTC takes small organic molecules dissolved in water and by transfer to a Teflon-lined autoclave at high temperature CQDs are produced.
A suitable photocatalytic system will be selected by spectral matching with the designed waveguide. A successful photocatalyst for homogenous CO2reduction must display adequate light harvesting, rapid charge separation and active catalytic sites. Efficiency is facilitated by sufficiently fast charge transfer/ migration, efficient photon utilisation and multiple active sites capable of surface absorption of CO2. There are a variety of photocatalysts that are of interest namely transition metal complexes (Ru, Re, Ir, Ni, Fe, Co, Cu Mn), plasmonic metal nanoparticles (Au, Ag) and metal-organic frameworks (MOFs).
George Rudman – Understanding Ion Mobility Mechanisms in Solid Electrolytes
Supervisor: Dr Karen E. Johnston and Dr James Dawson
The reduction of greenhouse gas emissions via the implementation of new and efficient technologies is a necessary step in addressing the current global climate crisis. The electrification of transport and the development of large-scale energy storage will contribute significantly towards net-zero emission goals. However, current commercial batteries, which are dominated by lithium-ion (Li-ion) technology, cannot meet the next-generation requirements. Therefore, the creation of new low cost, high-performance batteries is an essential goal for researchers today. Solid-state batteries are widely regarded as the mechanism for delivering future improvements in both the performance and safety of batteries. Current Li-ion batteries use flammable organic liquid electrolytes, thereby making them unsafe owing to the potential risks of electrolyte leakage. In contrast, all-solid-state batteries, i.e., those employing a solid electrolyte material, are less likely to explode or ignite if the battery is damaged. In addition, solid electrolyte materials can offer improvements in cost, long term stability and ionic conductivity, enabling cheaper and more efficient batteries with a longer life span to be produced. Numerous classes of materials have been proposed as possible solid electrolyte materials, including oxides with garnet, LIthium Super Ionic CONductor (LISICON) and perovskite structures. One promising class of materials are lithium-rich anti-perovskites (LiRAP’s), such as Li3OCl, which have supposedly shown superior ionic conductivity and lower activation energies over other potential candidate materials. However, LiRAP’s are highly hygroscopic and therefore require synthesis under an inert, moisture-free atmosphere. Li3OCl has proven to be difficult to synthesise and, as such, there is considerable uncertainty as to whether it exists as a thermodynamic, metastable or kinetic phase. I will begin my research by attempting the synthesis of Li3OCl using a range of air-sensitive techniques (Schlenk line and Glovebox synthesis) and conventional solid-state synthesis methods. A series of doped LiRAP’s will then be investigated, starting with fluorine doping, which has been reported to demonstrate an increase in ionic conductivity, but with limited explanation of why this phenomenon might occur. Therefore, investigations will be carried out to determine the influence of fluorine doping on the ionic conductivity. A combination of X-ray and neutron powder diffraction and solid-state NMR spectroscopy will be used to characterise the materials prepared. Atomistic modelling and density functional theory (DFT) calculations (ab initio molecular dynamics) will be carried out in conjunction with experimental methods in order to gain further structural insight and understanding. It is envisaged that, as the project progresses, additional Li- and/or Na-based anti-perovskite solid electrolyte compositions will also be investigated. Throughout the project I hope to develop a comprehensive understanding of the structure and ion conduction mechanisms for the different solid electrolyte materials modelled and synthesised, provide a scientific justification for the increased ionic conductivity due to fluorine doping, as well as determine the compatibility of the solid electrolytes within different solid-state battery systems.
Udari Wijesinghe – Nano-ribbon solar fuel devices
Supervisor: Dr Oliver Hutter
Energy generated from environmentally friendly, cost-effective solar cells offers a sustainable solution to meet the increasing global energy demand. Hence, researchers are focused on the development of thin-film solar cells using highly efficient, environmentally friendly, earth-abundant absorber materials like metal sulfides and selenides. Compared to other emerging compounds, antimony selenide (Sb2Se3) has gained tremendous interest as a promising photoactive material in photovoltaics due to its advantages of simplified phase chemistry, high physiochemical stability, suitable bandgap, high carrier mobility, and high absorption coefficient.
Different physio-chemical methods are used to generate Sb2Se3 thin films, which include rapid thermal evaporation (RTE), magnetron sputtering, close-spaced sublimation, electrodeposition, atomic layer deposition, etc. When the Sb2Se3 thin films are deposited onto a substrate, the (Sb4Se6)n ribbons exhibit different growth orientations (lateral and vertical growth modes) which are difficult to control due to their complex microstructures. In the (Sb4Se6)n 1D chain structure, the atoms are covalently bonded, whereas ribbons are interconnected by van der Waals forces. This results in faster migration of carriers along (Sb4Se6)n ribbons than between ribbons. In order to provide improved charge transport and to reduce the charge recombination throughout the Sb2Se3 layer, the ribbons should oriented perpendicular to the substrate. Therefore, it is crucial to regulate the ratio of lateral and vertical growth of (Sb4Se6)n ribbons in Sb2Se3 thin films to ensure efficient carrier transport. Thus, this study focuses to explore novel growth conditions for optimal Sb2Se3 nanoribbon orientation for efficient solar cells.
In order to analyze the preferred orientation of 1D nanoribbons, the Sb2Se3 will be deposited onto the fluorine-doped tin oxide (FTO) substrates with the use of RTE. To understand the growth mechanism and carrier transport behavior of Sb2Se3 thin films, the structure, and properties of Sb2Se3 thin films will be systematically characterized and analyzed by X-ray diffraction, scanning electron microscopy, transmission electron microscopy, surface profilometer, and Kelvin probe force microscopy.
Moreover, Sb2Se3 thin films on solar cell performance will be investigated. Herein the thin-film solar cells devices are configured in superstrate configuration where the thin film is coated with a buffer layer and then ends with the deposition of metallic back-contact (i.e., Au). The buffer used in this study to form a p-n heterojunction with an absorber layer is n-type TiO2. The photo-generated electrons then move from Sb2Se3 (p-type) to TiO2 (n-type) due to the creation of a built-in electric field at the p-n heterojunction between the Sb2Se3/TiO2 interface. On the other hand, the photo-generated holes are attracted by the p-type hole-transport layer and collected by the Au which will reduce the recombination at the back contact. Finally, the effect of the modified thin films will be used to fabricate efficient water splitting devices.
Ruth Pollard – Fluorotonix: Fluorescence standards to characterise the photoluminescence quantum yield of molecules for photovoltaic and display applications
Supervisor: Dr Marc Etherington
Research Vision
Renewable energies technology is to play a massive part in the future of energy generation especially with the global concern of climate change. Increasing the efficiency of organic light-emitting diodes (OLEDs) is one focus in striving for a greener future. More than 20% of the world’s energy consumption is due to lighting and displays and developing more efficient materials for OLED applications will help reduce this usage and save energy. A key goal in the development of OLEDs is to produce stable systems that have 100% internal quantum efficiency (IQE) i.e. every charge injected into the device creates a photon. The original OLEDs were limited to 25% IQE due to the quantum mechanical nature of the charges but recent developments allow us to push beyond this limit. Thermally activated delayed fluorescence (TADF) is one of those developments and will be a main focus on our journey to achieve 100% IQE.
The principal focus of my project is optimising organic compounds in the blue region. This is particularly critical as high energy blue emission is a difficult region to obtain stable TADF compounds. Commercial blue OLEDs are often based on emitters that undergo triplet-triplet annihilation; a different phenomenon that is limited to maximum 62.5% IQE. Finding a stable, blue TADF emitter of 100% IQE will have significant efficiency gains on the current state-of-the-art.
Approach
N-alkylation of donor – acceptor organic compounds: Working with collaborators at the University of Glasgow I will characterize groups of quinoline and pyridine salts with carbazole moieties and differing counter ions in terms of their photoluminescent properties. The Etherington group has begun to study the effect of N-alkylation and quaternisation with these compounds and my project will aim to develop and probe the excited state processes that occur from this. I will analyse how N-alkylation tunes the compounds such that TADF can be activated. The study therefore provides a systematic way to achieve the stable blue light-emitting materials desired by commercial companies.
N-alkylation of natural products: Working with Dr Jonathan Knowles in Applied Sciences at Northumbria University I will probe how N-alkylation of organic compounds, for natural product like compounds such as quinine. This will allow us to understand how the synthetic procedure affects the more fundamental processes such as charge transfer and conjugation that lead to the macroscopic phenomena such as TADF.
Methods: These compounds will be measured both in solution and solid form, across a series of host environments and the preparation method of film samples will also be investigated by comparing the drop cast and spin coating methods. I will obtain their photoluminescence quantum yield (PLQY), which defines a measure of the efficiency of photon emission as a ratio of photons emitted and photons absorbed and is a crucial property of these compounds if they are to be of commercial use. A detailed understanding of PLQY, steady-state and time-resolved photoluminescence is key for these systems and will provide both fundamental knowledge and also the criteria that are of interest to commercial OLED companies.
More efficient displays mean reduced energy consumption
The world is now heavily reliant on portable electronics and mobile phones. Increasing the efficiency of their displays is a crucial way to reduce energy consumption in these devices. Understanding the physical processes that can improve their efficiency puts this work at the core of EPSRC’s remit. This project will allow me to use my chemical background and explore the physical properties of materials for OLEDs, making this a truly interdisciplinary project across the physical sciences. It will combine a fundamental and applied approach to organic light-emitting materials and will give me the opportunity to train on and compile data from a wide range of equipment at the universities of Northumbria and Durham.
Tida Moyo – Droplet kinetic energy harvesting for advanced engineering applications in IoT
Supervisor: Dr Yifan Li
The first law of thermodynamics states ‘that energy cannot be created or destroyed: it can only change form’ as humanity steers away from the resources that have been damaging our environment and the planet. We have demanded a lot more from the tools that we have at our disposal. 5g technology has made the grand vison of ‘the internet of thing’ (IoT) closer to reality than ever. However, this demand is opposed by the current limitations in powering the sensors that can push us into this IoT future. Their maintenance, miniaturisation, and recyclability limits batteries for sensing.
In recent years, there has been a big focus on Nano-energy harvesting in the scientific community, with the rise of piezoelectric, thermoelectric and the triboelectric technology. Energy harvesting from Reverse electrowetting on Dielectric (REWOD) (Wu, 2020),and rain droplet energy harvesting are promising ways of powering sensor technology in a sustainable and low maintenance way. The amount of energy that can be harvested by a REWOD system is proportional to the capacitance of the system. There are several ways of doing this, each with their own hurdles that need to be overcome.
The aims of this project firstly to maximise the to maximise the amount of energy that can be harvested by the REWOD system and other energy harvesting systems like Triboelectric nano generators (TENGS) (Renyun Zhang, 2021) ,by investigating new techniques that can increase the efficiency of these processes. Fresh approaches to the characterisation that allow for tailored surface design will also be beneficial to developing materials with optimal characteristics. Second, the to investigate the integration of multiple energy harvesting technologies in order to maximise energy generation under any conditions.
New studies conducted on the interactions between droplets and hydrophobic substances have unlocked new techniques that have the potential to further increase the energy harvesting efficiencies while simultaneously decreasing the footprint of the energy harvesters.
In addition, the research will also investigate the possibility of technology integration. For example, high-solar transmittance thin films in REWOD and TENG rain drop energy harvesting, integrable with photo-voltaic (PV) devices, promoting a new generation of sensors self-powering in either rain or sunshine.
Research into other ‘waste’ energy harvesting will be conducted to aid in creating the smart IoT future. Some examples of this are reclaiming rain drops and thermal energy from the panel for freshwater production. These methods in conjunction, will help us propose a more sustainable way to power sensor nodes, no matter the time or the conditions.
The potential applications of this research will range from smart servicing the energy requirements for outdoor sensor nodes, the energy harvesting of human locomotion, energy reclamation in the automotive industry and everything in between.
Prakriti Kayastha – Modelling disorder in magnesium battery cathode materials
Supervisor: Dr Lucy Whalley
Atomistic modelling of materials offers great potential for predicting desirable properties before material synthesis in the laboratory. For example, we can probe hypothetical materials to find those with an optimum band gap for solar cell applications, or those that are able to efficiently transport metal ions for use as a battery cathode material.
During my PhD we will model energy materials using quantum chemical methods including plane-wave Density Functional Theory (DFT). In this method a bulk material can be modelled with a careful selection of the unit cell, which is then repeated in 3-dimensional space with periodic boundary conditions. Usually the real crystal is approximated as static and pristine (without any defects), which misses much of the rich physics and behaviour associated with thermal vibrations and atomic-scale imperfections. In this project, we will use lattice dynamics and defect physics to consider processes beyond this idealised model. We will use ab-initio packages running on Oswald (the Northumbria supercomputer) and Archer2 (the national supercomputer) to generate DFT data. The output will be analysed and post-processed using open source codes. We will also develop our own custom open-source codes for analysing data according to the needs of the project.
The PhD will begin by modelling chalcogenide perovskite materials, an emerging class of materials that are being developed for use in optoelectronic devices. These materials are proposed to be a good substitute for the popular albeit toxic lead-based perovskites. In this project we will model the zirconium based chalcogenide perovskite, BaZrS3, working in collaboration with experimental colleagues who are synthesising the compound using ball-milling. DFT modelling will determine the structure and thermal properties of the material. A further analysis of the vibrational modes will produce the IR and Raman characteristics of the phonon modes to compare with the experimental results. A detailed analysis of the reactants, intermediates and the by-products produced during the synthesis of the perovskite will introduce more insight into the reaction mechanism and the relative stability of each of the compounds.
With the skills and experience acquired during the lattice dynamics project we will move onto modelling cathodes for multivalent batteries. As the inherent limitations of monovalent, lithium-ion based batteries are being realised, and as lithium reserves are becoming depleted, new candidates with a higher theoretical energy density are being proposed. We will the study magnesium based spinel compounds. Usually pristine structures of crystals are modelled using DFT, whilst any chemical disorder or defects are completely neglected. However the process of charging and discharging leads to unavoidable disorder in battery materials, which in turn determines the energetics of defect formation and material stability. With current DFT methods, the fully charged and discharged cathodes can be readily modelled, whilst relatively little is known about the intermediate processes that take place during the charge cycle. We will use a cluster expansion techniques to model disorder and predict material performance through the battery charge cycle.
This project will explore various computational techniques, such as designing automated workflows, high-throughput DFT evaluation, development of post-processing codes and machine learning. We will navigate through different crystal systems and establish structure-property relationships, predicting the bulk, defect and transport properties of emerging energy materials. These insights, developed throughout the course of my PhD, will bring the scientific research community one step closer to meeting the challenges of Net Zero.
Alexis Aguilar Celis – Decentralised Integration of Renewable Energy sources through smart grid Technologies Supervisor: Dr Hongjian Sun
The purpose of this project is to understand how energy technologies made from new materials (PVs, batteries etc) influence the operation of smart grids. In particular, this project will examine the impact of new PV materials and technologies on the power grid, e.g., considering different PV yield in low light conditions and Tandem/hybrid cells (organic, inorganic or hybrid) expecting a change in behaviour leading to a better power grid stability.
This project will make usage of Durham Smart Grid Laboratory which has two real-time digital simulators (RTDS) and renewable energy emulators (wind/solar emulators). We will be using energy network existing data and focus on coordinating renewable energy with energy storage taking advantage of recorded sensor data. This includes the usage of machine learning techniques to have a better understating of the virtual power plants implications and results.
Matin Ataei Kachouei – Development of a Novel Ammonia Fuel Cell to Reduce Particulate Matter (PM) Impactful of Ammonia Emissions from UK Agriculture Supervisor: Dr. Maryam Bayati, Dr. Mohamed Mamlouk, and Prof. Ulrich Stimming
Air pollution is the top environmental risk to human health in the United Kingdom, and the fourth greatest threat to public health after cancer, heart disease and obesity. Unlike other air pollutants, ammonia emissions in the United Kingdom have been rising since 2013, with significant implications for biodiversity, human and livestock health, and environment. Once ammonia mixes with other gases in the atmosphere, it can form particulate matter (PM) with major health concerns to the lungs and heart. Besides, agriculture emitted around 244900 tons ammonia in 2017. The government`s aim is to reduce emissions of ammonia against the 2005 baseline by 16% by 2030 and to reduce PM emissions by 46% by 2030. Ammonia is a hydrogen vector and can work as an important player toward net zero plan.
This project aims to develop an innovative and versatile electrochemical fuel cell for ammonia electro-oxidation (AEO) that benefits eliminating ammonia as well as generating clean electricity as a promising step toward Net-Zero Carbon goal. This mean providing clean energy rather than using fossil fuels to provide it. In this regard, the whole project will divide into several section that each step tends to address one of the main challenges during this project, as it is shown in Figure 1.

AEO is one of the most prominent electrochemical reactions having great potentials to solve severe energy and environmental problems. In the first step, which is considered as the core of this project, novel nanoparticulated electro-catalysts using earth-abundant material will be prepared based on theoretical calculation. In general, the electro-oxidation of ammonia uses a metal catalyst, such as platinum, copper, or nickel, their composites, or alloys. Previous studies demonstrates that a combination of heterogeneous metallic catalysts could improve the performance of the AEO. In fact, this is the synergistic effect among these heterogeneous metallic centers that improves the performance of this electro-catalyst. Therefore, the aim of this project is to prepare bi or tri-metallic catalyst. The mechanism of the AEO will be thoroughly investigated by employing a combination of spectroscopic, microscopic and electrochemical techniques. Finally, by considering all of the variables the cell device will simulate and design to attain maximum performance. The fabricated fuel cell will be tested in site for ammonia mitigation. This fuel cell can aid in the delivery of Net Zero Agriculture in the livestock sector which means every farm is offsetting their ammonia emissions.
Alex Bell – Understanding Electrode-Electrolyte Interfaces for Next-Generation Batteries Supervisor: Dr James Dawson and Dr Karen Johnston
The production capacity of batteries is set to rise drastically within the coming decades, precipitated by the projected expansion in renewable (yet intermittent) power generation, the upcoming bans on diesel and petrol engines that are driving a rapid increase in electric car manufacture, and the permeation of decentralised power networks, among many other influences. The scale of resource extraction required to furnish such storage needs necessitates the development of a new generation of batteries with improvements in sustainability, performance, and safety characteristics over current battery technologies.
The substitution of conventional liquid electrolytes (LEs) with novel solid electrolytes (SEs) in glassy, ceramic, and other polycrystalline phases has shown promise in addressing these requirements. Potential SEs have demonstrated characteristics such as cycling stabilities on the order of supercapacitors, high room temperature ionic conductivities, wide electrochemical windows, and improved safety under compromisation of cell structure (when electric cars crash, for example) due to their thermodynamic stability in air. Despite these advantages, the incorporation of SEs into operational, all-solid-state batteries (ASSBs) presents a myriad of constraints to be mitigated. For example, where LEs enable fast interfacial kinetics due to a high degree of electrode wetting, SE-electrode interfaces induce a rate-limitation due to high resistance, thereby inhibiting ion transport. This high resistance is considered to originate owing to crystal lattice mismatch, poor interfacial contact, ion-deficient space-charge layers, and interphase formation. Hence, such complex challenges, amongst many others, make finding suitable SE materials a highly non-trivial task.
To fully optimise these candidate materials as electrolytes, a comprehensive understanding of the interfacial and bulk ion dynamics is needed. However, owing to the buried nature of interfaces and transport mechanisms, the direct observation of ion mobility is difficult and the complete dynamic picture, which is crucial to the design of high-performance SSBs, therefore remains elusive. In this vein, this project aims to elucidate the structural motifs responsible for improved ionic conductivity; carefully combining results from materials synthesis and characterisation with those from density functional theory (DFT) calculations and ab initio molecular dynamics (AIMD) simulations.
One family of materials hosting many promising candidate SEs are sulphides, where the polarisability of the sulphide anion provides a low electrostatic barrier to ion migration, with, for example, Li10GeP2S12 (LGPS) and Li6PS5Cl showing room temperature ionic conductivities on the order of, and surpassing, many organic LEs. Recent work on lithium-boron-sulphur-type systems suggest better ionic conductivities and electrochemical stabilities than LGPS, and it is within this family where this research project will begin. The effects of different synthetic methods and compositional doping on structure and the resulting conductivity of candidate SEs will be explored using a combination of X-ray and neutron powder diffraction with multinuclear solid-state NMR spectroscopy. It is anticipated that DFT and AIMD calculations will assist in elucidating ion mobility mechanisms in such systems, and provide valuable insights into potential optimisation routes for future high-performance SE materials and interfaces.
Catherine Crockett – Reducing Environmentally Problematic Landfill And Circular Economy approaches for Waste Minerals (REPLACE for Waste Minerals) Supervisor: Prof. Chris Greenwell
Much of what society depends on in the modern day has its roots in fossil fuels, including electricity, transport, heating, and plastic production. However, fossil fuels are a finite resource with coal, gas and oil projected to reach depletion within 42 years’ time (New York Circular City Initiative, https://www.circularnyc.org/). In addition to fuels, we mine and consume a large amount of metals and minerals, which are also non-renewable resources. This may seem a less urgent situation until we realise that our smartphones are laden with rare earth metals, many of which are running out.
What if we could recover metals from waste we produce, waste which would otherwise have been put into landfill, and, furthermore, transform it into something useful? This will form part of my project to develop circular economy approaches, addressing the problem of our current and mostly linear economy. I will be transforming struvite, a problematic waste mineral produced as a consequence of waste-water treatment (WWT), into industrially useful layered double hydroxide (LDH) materials. The magnesium within the struvite will be a metal precursor for the LDH synthesis. I will aim to synthesise these using green chemistry principles and utilising various sources of waste throughout.
LDH’s have potential applications in many different fields. Here, I will focus on their role as catalysts in reactions such as biofuel and green hydrogen production, as well as carbon capture and transformation. Furthermore, I will try and design the synthesis and use of the LDH with Northumbrian Water Groups’ WWT plant in mind, creating a circular economy approach in which the waste they produce has been transformed to something they can once again use. This will help the company attain its net zero waste target.
A novel approach to LDH synthesis is being taken in this project, alongside the use of the LDH to create renewable resources and circular economy pathways.
Thomas Parker – Earth Abundant Transparent Conductors for Next Generation Photovoltaics Supervisor: Dr Iddo Amit, Prof Dagou Zeze
The international goal of keeping global temperature rise to well below the 2 degrees Celcius threshold requires accelerating the uptake of low-carbon and renewable methods of energy generation. It is expected that Photovoltaic technology, having already demonstrated notable industry success, will make up a considerable proportion of this new energy mix. The rate of uptake of this technology has been considerable, but a number of challenges remain to further accelerating sector growth.
A key consideration in the development of Photovoltaic technology is the technology used as the top electrode. Commercial Silicon cells have typically used silver nanowires for this purpose, which boast a high conductivity but introduce shotkey junctions and parasitic absorption, both hampering performance. An alternative often present Thin-Film applications across Optoelectronics is Sn-doped Indium Oxide (ITO), a highly conductive and optically transparent semiconductor. Although robust and amenable for deposition this technology suffers from the associated high cost of Indium, often providing a limiting factor on the overall cost of the device. There are also a number of ethical concerns over the extraction of Indium which must be considered as we attempt to make the green transition an ethical one.
While a number of alternative such thin-film transparent conductors exist, there has been very little transition away from ITO as the dominant material in the industry, partly due to issues of reliability and the cost of a transition. Many alternative materials, such as other post-transition metal oxides, lack ITO’s consistency across many applications. However, there is also substantial difficulty in choosing potential materials to investigate, as the bulk characteristics of these materials are often very different to that of their thin-film and polycrystalline phases, leading to quite different charge-transport behaviour.
My project is therefore pursuing methods to quantify the electrode characteristics of candidate transparent conductors. This will involve development of statistical models of charge transport through polycrystaline semiconductor thin-films, and experimental measurement (AFM/KPFM, SEM) of the structure of candidate transparent conductors, to help determine their potential for use in next-generation photovoltaics.

